Description
Core advantages:
High purity: We use advanced production technology to ensure product Purity ≥ 99.8%, Impurities < 0.1%, providing reliable guarantee for your production and experiments. ”
Stable quality: Strictly implement ISO9001 quality management system, each batch of products undergoes 36 rigorous tests to ensure stable performance. ”
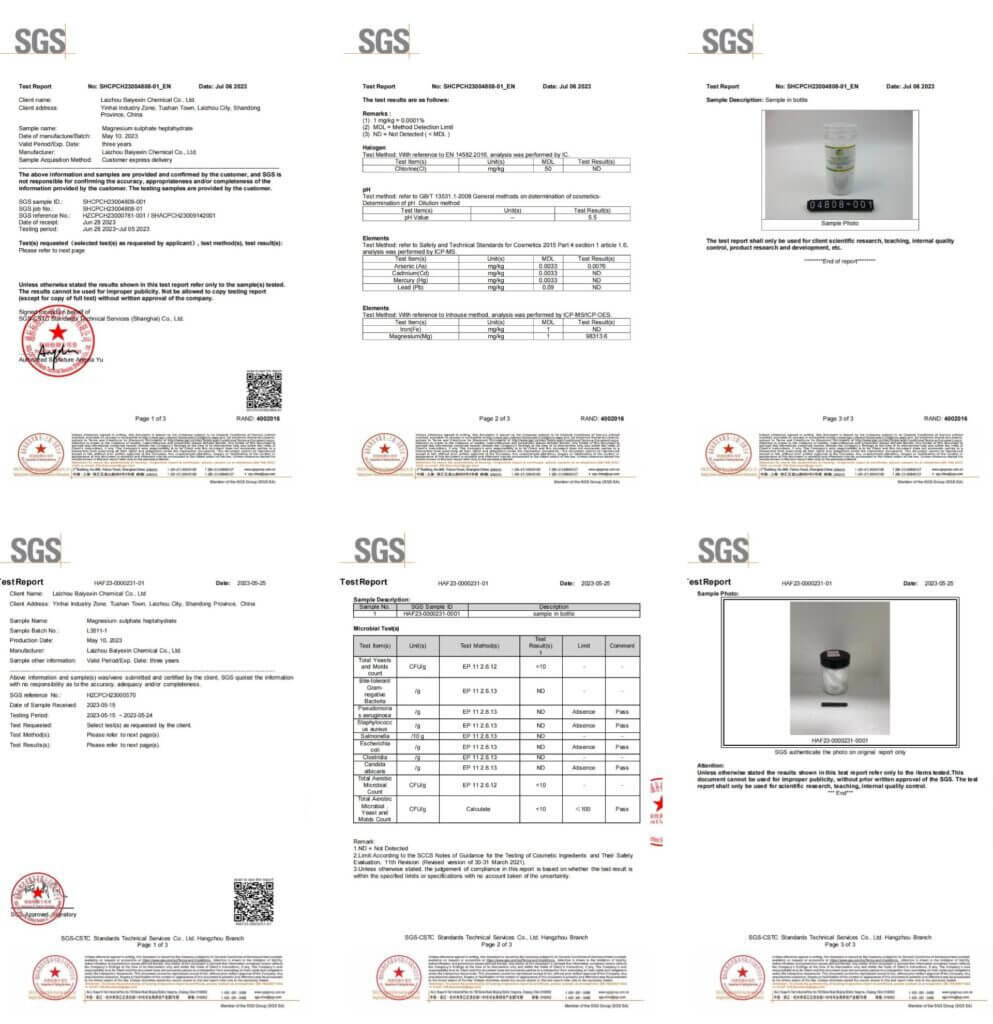
Magnesium Sulfate Anhydrous Examining Report:
PACKING: 25kg/50kg/1000kg/1250kg:

Certification:

Factory Picture:

As a core member of the magnesium sulfate family, magnesium sulfate anhydrous, distinguished by its lack of crystal water, holds an irreplaceable position in fields such as desiccants, pharmaceuticals, industry, and agriculture due to its exceptional water absorption, stable chemical properties, and versatile functionality. Its fundamental differences from heptahydrate and monohydrate magnesium sulfate determine its unique application scenarios and technical requirements. This article provides a systematic analysis of magnesium sulfate anhydrous, covering its basic properties, preparation processes, applications, safety regulations, market landscape, and common questions, serving as a comprehensive technical and industrial guide.
Key Takeaways
- Basic Information: From Physical Properties to Core Characteristics
- Preparation Methods: Industrial Processes and Laboratory Techniques
- Applications: From Desiccants to New Energy Materials
- Safety and Storage: Risk Control and Standardized Operations
- Magnesium Sulfate Anhydrous Purity and Quality Control: Grade Standards and Identification Methods
- Market and Development: Global Landscape and Trends
- Magnesium Sulfate Anhydrous Packaging Specifications
Properties and Functions of Magnesium Sulfate Anhydrous
1. Basic Information: From Molecular Structure to Core Properties
1.1 Name, Identifiers, and Molecular Characteristics
Chemical Name: Magnesium Sulfate Anhydrous
Other Names: Anhydrous Epsom Salt, Magnesium Sulfate Anhydrous Powder, High-Purity Magnesium Sulfate Anhydrous, Magnesium Sulfate Anhydrous Granules, Magnesium Sulfate Anhydrous 99.5%
CAS Number: 7487-88-9
EINECS Number: 231-298-2
Chemical Formula and Molecular Weight: MgSO₄, molecular weight 120.37 g/mol (compared to 246.47 g/mol for heptahydrate magnesium sulfate and 138.38 g/mol for monohydrate magnesium sulfate, the key difference being crystal water content).
Molecular Structure and Spatial Arrangement:
Magnesium sulfate anhydrous is an ionic crystal, formed by strong ionic bonds between Mg²⁺ and SO₄²⁻. Its spatial structure is a face-centered cubic lattice, with SO₄²⁻ forming a tetrahedral framework and Mg²⁺ occupying interstitial spaces, devoid of crystal water molecules in the lattice. This structure results in a high crystal density (2.66 g/cm³) and strong lattice stability, requiring high temperatures for decomposition.
Comparison with Hydrated Forms:
The differences between magnesium sulfate anhydrous, heptahydrate magnesium sulfate (MgSO₄·7H₂O, CAS: 10034-99-8, MW: 246.47 g/mol), and monohydrate magnesium sulfate (MgSO₄·H₂O, CAS: 14168-73-1, MW: 138.38 g/mol) are primarily due to.
Hydration Level: Anhydrous form has no crystal water, heptahydrate has seven water molecules, and monohydrate has one, leading to differences in molecular weight and stability.
Appearance and Stability: Anhydrous form is a white powder or granules, highly hygroscopic; heptahydrate appears as transparent crystals, more stable; monohydrate is a white powder or crystal, intermediate in stability.
Solubility and Applications: Anhydrous form excels in water absorption, ideal for desiccants; heptahydrate is used in pharmaceuticals and fertilizers; monohydrate serves as an industrial intermediate.
Thermal Stability: Anhydrous form decomposes at 1124°C; heptahydrate loses water at 150°C; monohydrate loses water at 120°C.
Table: Comparison of Basic Properties of Anhydrous and Hydrated Magnesium Sulfate
| Property | Magnesium Sulfate Anhydrous | Heptahydrate Magnesium Sulfate | Monohydrate Magnesium Sulfate |
| Chemical Formula | MgSO₄ | MgSO₄·7H₂O | MgSO₄·H₂O |
| Molecular Weight | 120.37 g/mol | 246.47 g/mol | 138.38 g/mol |
| Appearance | White powder or granules | White, fine prismatic or columnar crystals | White crystals or powder |
| Magnesium Content | 20.2% | ~9.9% | ~17.4% |
| Main Applications | Desiccants, organic synthesis | Pharmaceuticals, agricultural fertilizers | Feed additives, industrial applications |
1.2 Physical Properties: From Appearance to Key Physicochemical Parameters
Appearance and State: At room temperature, magnesium sulfate anhydrous is a white powder or colorless orthorhombic crystal. Granular products (particle size 0.1–3 mm) are prepared via granulation processes, odorless with a slightly bitter taste.
Density: Crystal density is 2.66 g/cm³ (20°C); loose powder density ranges from 0.8–1.0 g/cm³ (varies with particle size).
Melting and Boiling Point: Melting point is 1124°C (decomposes into MgO, SO₃, and SO₂ upon melting); no true boiling point (continuous decomposition at high temperatures).
Water Absorption: Highly hygroscopic, with a water absorption rate of ~0.1 g/(g·h) at 25°C and 60% relative humidity (RH), capable of absorbing 1.05 times its weight in water, ultimately forming heptahydrate magnesium sulfate.
Solubility:
Water: Highly soluble, with a solubility of 35.1 g/100 mL at 20°C, exothermic upon dissolution (enthalpy of dissolution ΔH = -84.9 kJ/mol).
Organic Solvents: Slightly soluble in ethanol (<0.5 g/100 mL) and glycerol; insoluble in non-polar solvents like acetone, ether, and benzene.
Refractive Index: Crystal refractive index n₂₀/D = 1.56; powder lacks clear refractive index data due to scattering.
Hygroscopicity: Extremely hygroscopic, begins to clump within 1 hour in environments with RH > 40%, requiring sealed storage.
1.3 Chemical Stability and Valence
Stability at Ambient Conditions: Stable under normal temperature and pressure, does not react with O₂ or CO₂ in air but readily absorbs moisture, transforming into monohydrate or heptahydrate (from powder to clumps).
High-Temperature Stability: Stable up to 200–250°C; decomposes above 1124°C via:
Main reaction: MgSO₄ → MgO + SO₃↑
Side reaction: 2SO₃ ⇌ 2SO₂ + O₂
Reaction with Water: Not a chemical reaction but a physical adsorption and lattice reconstruction process, forming hydrated compounds (MgSO₄ → MgSO₄·H₂O → MgSO₄·7H₂O), which is exothermic.
Reactions with Acids, Bases, and Salts:
- With strong bases (e.g., NaOH): Forms white Mg(OH)₂ precipitate: MgSO₄ + 2NaOH → Mg(OH)₂↓ + Na₂SO₄
- With soluble barium salts (e.g., BaCl₂): Forms BaSO₄ precipitate (characteristic test for sulfate ions).
- No reaction with acids (as it is a salt of a strong acid, lacking weak acid ions).
Valence Composition: Identical to heptahydrate magnesium sulfate—Mg: +2, S: +6, O: -2; only the absence of crystal water differentiates them.
1.4 Water Absorption Mechanism and Desiccant Performance Comparison
Water Absorption Mechanism: The hygroscopicity of magnesium sulfate anhydrous stems from "lattice vacancy filling" and "ionic polarity adsorption":
- Its ionic crystal structure contains unoccupied lattice vacancies, allowing water molecules to enter and form coordination bonds with Mg²⁺ (a highly polar cation), gradually forming hydrated compounds.
- The strong polarity of Mg²⁺ and SO₄²⁻ creates high adsorption energy on the crystal surface, enabling physical adsorption of water molecules from the air, which then convert into crystal water.
Depending on environmental temperature and humidity, it sequentially forms hydrates (e.g., monohydrate, trihydrate, up to heptahydrate). This process is reversible; heating can remove crystal water to regenerate the anhydrous form. Theoretically, 1 mol of magnesium sulfate anhydrous can absorb 7 mol of water (126 g water/120.37 g ≈ 105%).
Comparison with Common Desiccants:
| Desiccant | Water Absorption (25°C, RH 60%) | Applicable Solvents/Gases | Corrosivity | Regeneration Temperature | Cost (Relative) |
|---|---|---|---|---|---|
| Magnesium Sulfate Anhydrous | 1.05 g/g | Organic solvents, neutral gases | Low | 200–250°C | 1 |
| Anhydrous Calcium Chloride | 0.75 g/g | Polar gases, hydrocarbons | High (corrodes metal) | 150–200°C | 0.8 |
| Molecular Sieve (3A) | 0.25 g/g | Polar molecules (water, ethanol) | None | 300–350°C | 3 |
| Anhydrous Sodium Sulfate | 0.15 g/g | Weakly polar organic solvents | Low | 180–220°C | 0.9 |
Advantages: magnesium sulfate anhydrous has stronger water absorption than most desiccants except calcium chloride, with low corrosivity, making it suitable for moisture-sensitive scenarios (e.g., electronics).
2. Preparation Methods: Industrial Processes and Laboratory Techniques
2.1 Industrial Core Process Routes
Industrial production of magnesium sulfate anhydrous aims for low cost, high purity, and low energy consumption, with three main routes:
1. Dehydration of Heptahydrate/Monohydrate Magnesium Sulfate (Mainstream, 70% Share)
- Raw Materials: Industrial-grade heptahydrate magnesium sulfate (MgSO₄·7H₂O ≥ 98%) or monohydrate (MgSO₄·H₂O ≥ 99%).
- Core Steps:
- Pretreatment: Pulverize raw material (particle size < 1 mm), wash with deionized water to remove impurities (e.g., calcium, chloride), and centrifuge to reduce water content (< 5%).
- Dehydration: Two-stage process:
- Stage 1: Dry at 100–150°C with hot air to remove free water and some crystal water, yielding monohydrate.
- Stage 2: Calcine at 200–250°C (or vacuum dehydrate at -0.095 MPa) to remove all crystal water, yielding magnesium sulfate anhydrous.
- Post-processing: Cool (to prevent moisture absorption), pulverize/granulate, sieve, and package in sealed containers.
- Reaction Conditions: Dehydration temperature must be controlled at 200–250°C (below 200°C, incomplete dehydration; above 250°C, particle sintering occurs).
- Raw Material Requirements: Calcium content ≤ 0.3%, chloride ≤ 0.1%; otherwise, additional impurity removal (e.g., Na₂CO₃ for calcium, BaCl₂ for excess sulfate) is needed.
2. Direct Reaction of Magnesium Oxide and Sulfuric Acid (High-Purity Products, 20% Share)
- Raw Materials: Light magnesium oxide (MgO ≥ 95%), industrial-grade sulfuric acid (H₂SO₄ ≥ 93%).
- Core Steps:
- Reaction: Add deionized water to a reactor, slowly add sulfuric acid, then add MgO in batches, controlling pH at 6–7 to avoid excess MgO. Reaction: MgO + H₂SO₄ → MgSO₄ + H₂O.
- Purification: Filter the reaction solution to remove unreacted MgO and impurities, concentrate by evaporation (to 40%–45%), and cool to crystallize monohydrate magnesium sulfate.
- Dehydration: Vacuum dry monohydrate at 200–250°C to obtain magnesium sulfate anhydrous (purity ≥ 99.5%).
- Advantages: High purity, suitable for pharmaceutical and reagent-grade needs.
- Disadvantages: High raw material costs (MgO is three times more expensive than heptahydrate) and higher energy consumption.
3. Salt Lake Brine Concentration (Resource-Based, 10% Share)
- Raw Materials: Salt lake brine (Mg²⁺: 20–30 g/L, SO₄²⁻: 30–40 g/L, e.g., Qarhan Salt Lake, China).
- Core Steps:
- Concentration: Evaporate brine in solar ponds (to MgSO₄ concentration ≥ 200 g/L), remove impurities (NaOH for Fe³⁺/Al³⁺, BaCl₂ for excess SO₄²⁻).
- Crystallization: Cool concentrated solution to precipitate sodium chloride, then further concentrate to precipitate heptahydrate magnesium sulfate.
- Dehydration: Calcine heptahydrate at 200–250°C to obtain magnesium sulfate anhydrous.
- Advantages: Low raw material cost, environmentally friendly (uses renewable solar energy).
- Disadvantages: Limited to salt lake regions, variable product purity requiring multiple purifications.
Comparison of Dehydration Processes:
| Process | Energy Consumption (kW·h/t) | Product Purity | Crystal Form | Environmental Impact | Applicable Scenarios |
|---|---|---|---|---|---|
| High-Temperature Calcination | 800–1000 | 98%–99% | Powder | Moderate (requires flue gas treatment) | Industrial-grade products |
| Vacuum Dehydration | 1200–1500 | 99%–99.5% | Fine crystals | Excellent (no flue gas) | Pharmaceutical, reagent-grade products |
| Spray Drying | 1500–1800 | 98%–99% | Microspheres | Excellent (heat recyclable) | Granular products (e.g., desiccants) |
| Microwave Drying | 1000–1200 | 99%–99.5% | Powder | Excellent (no pollutants) | Laboratory small-scale preparation |
Current Mainstream Process: High-temperature calcination (heptahydrate dehydration), chosen for low cost (30% lower energy than vacuum dehydration), high capacity (up to 50,000 tons/year per line), and suitability for industrial-grade needs (98%–99% purity).
2.2 Laboratory Preparation and Impurity Control
Laboratory Preparation (from Heptahydrate Dehydration):
- Take 100 g analytical-grade heptahydrate magnesium sulfate (MgSO₄·7H₂O ≥ 99.5%) and place it in a porcelain crucible.
- Heat in a muffle furnace at 100°C for 1 hour to remove free water, then at 220°C for 2 hours to remove crystal water.
- Cool below 50°C in the furnace, then quickly transfer to a desiccator to prevent moisture absorption, yielding magnesium sulfate anhydrous (purity ≥ 99.8%).
Key Considerations:
- Temperature Control: Do not exceed 250°C to avoid particle sintering, which affects solubility.
- Moisture Prevention: Cooling and transfer must occur in a dry environment (e.g., glovebox), with storage in a vacuum desiccator.
Impurity Sources and Control:
- Sources: Raw materials (Ca²⁺, Fe³⁺, Cl⁻ in heptahydrate), equipment (Cr³⁺ from stainless steel reactors), process water (Na⁺).
- Control Methods:
- Raw Material Pretreatment: Wash heptahydrate with 0.1 mol/L H₂SO₄ to remove Ca²⁺ (forms CaSO₄ precipitate).
- Equipment Selection: Use titanium alloy reactors for pharmaceutical-grade products to avoid metal ion leaching.
- Crystallization Purification: Recrystallize with deionized water to reduce Cl⁻ content (≤ 0.001%).
Process Differences by Purity Grade:
- Industrial Grade (98%–99%): Single dehydration, no deep impurity removal.
- Pharmaceutical Grade (99.5%–99.8%): Uses MgO-H₂SO₄ reaction + vacuum dehydration + recrystallization.
- Reagent Grade (≥ 99.9%): Adds ion exchange resin purification to remove heavy metals (≤ 0.0001%).
3. Applications: From Desiccants to New Energy Materials
3.1 Core Application: Desiccants (40% Share)
Magnesium sulfate anhydrous ’s strong water absorption makes it the preferred neutral desiccant for scenarios sensitive to acidic or alkaline desiccants:
1. Organic Solvent Drying
- Applicable Solvents: Ethanol, ether, acetone, ethyl acetate, benzene, toluene (insoluble in these solvents, avoiding contamination).
- Usage:
- Add 5%–10% by weight of the solvent (e.g., 5–10 g per 100 mL ethanol).
- Stir for 10–30 minutes until the powder no longer clumps and the solution is clear, then filter.
- Precautions: Unsuitable for drying solvents containing amines or phenols (Mg²⁺ may coordinate with these compounds, causing adsorption).
2. Gas Drying
- Applicable Gases: Hydrogen, oxygen, nitrogen, carbon dioxide, methane (neutral or weakly acidic gases; avoid drying alkaline gases like ammonia to prevent Mg(OH)₂ formation).
- Usage:
- Use granular magnesium sulfate anhydrous(1–3 mm particle size to avoid dust clogging).
- Fill drying towers or tubes, with gas flow rates of 0.5–1 L/min (ensuring ≥ 5 seconds contact time).
- Standard: Dried gas dew point ≤ -40°C (complies with GB/T 14599-2016 for high-purity oxygen).
3. Pharmaceutical and Food Moisture Protection
- Pharmaceuticals: Used in packaging for tablets and capsules (e.g., 5–10 g in blister packs) to maintain humidity ≤ 30%, extending shelf life (e.g., for vitamin C, antibiotics).
- Food Preservation: Used in sealed packaging for baked goods (biscuits, bread) and nuts to absorb moisture and prevent mold (complies with GB 25588-2010 for food additives).
3.2 Pharmaceuticals (15% Share)
Oral Applications: Laxative and Choleretic Agent
- Mechanism: Not absorbed by the intestines, creates a hypertonic environment, preventing water absorption and stimulating peristalsis.
- Dosage: Due to high purity (MgSO₄ ≥ 99.5%), the dose is 50% of heptahydrate (adults: 2–5 g vs. 5–10 g for heptahydrate).
- Indications: Constipation, preoperative bowel cleansing, biliary colic (promotes bile excretion).
Topical Applications: Not used directly due to strong water absorption (may dry skin); compounded with glycerol (1:3 ratio) into ointments to relieve skin edema (e.g., from phlebitis).
Contraindications and Side Effects:
- Contraindications: Severe renal insufficiency (creatinine clearance < 30 mL/min), intestinal obstruction, acute abdomen.
- Side Effects: Overdose may cause dehydration, electrolyte imbalances (hypokalemia), or chronic intestinal dysfunction.
3.3 Industrial Applications (35% Share)
1. Flame-Retardant Materials
- Applications: Polyvinyl chloride (PVC), epoxy resin, polyurethane foam.
- Mechanism:
- Decomposes endothermically at high temperatures (MgSO₄ → MgO + SO₃), lowering material surface temperature.
- MgO forms a porous barrier, isolating oxygen.
- SO₃ dilutes combustible gases, suppressing combustion.
- Requirements: Added at 10%–20% of polymer weight, often compounded with synergists (e.g., aluminum hydroxide) to achieve UL94 V-0 rating (per GB/T 2408-2021).
2. Paper Industry
- Function: Used as a sizing aid with rosin to enhance paper water resistance.
- Mechanism: Mg²⁺ reacts with rosin acid to form magnesium rosinate, improving rosin adhesion to fibers and reducing water penetration.
- Standard: Complies with QB/T 4446-2012 (MgSO₄ ≥ 98%, water-insoluble matter ≤ 0.2%).
3. Metal Processing
- Rust Inhibitor: 5%–10% solution used to treat steel surfaces, forming a Mg(OH)₂ protective film, preventing rust for 1–2 months.
- Flux: Added at 0.5%–1% in aluminum alloy smelting to reduce melt viscosity and enhance impurity flotation, improving alloy purity.
4. Coatings Industry
- Filler Pigment: Added to latex paints (5%–8%) to improve flow and scrub resistance (from 2,000 to 5,000 scrub cycles).
- Anti-Corrosion Pigment: Used in metal primers with zinc powder to enhance adhesion and extend corrosion resistance.
3.4 Agriculture (8% Share)
- Characteristics: Magnesium is a core component of chlorophyll, critical for photosynthesis. Magnesium sulfate anhydrous provides a soluble magnesium source to correct soil magnesium deficiency, especially for magnesium-demanding crops (e.g., tomatoes, potatoes, roses, peppers) in magnesium-deficient soils (e.g., acidic red soils).
Comparison of Magnesium Fertilizers:
| Magnesium Fertilizer | Dissolution Rate | Absorption Efficiency | Applicable Soils | Cost (Relative) |
|---|---|---|---|---|
| Magnesium Sulfate Anhydrous | Fast | 85%–90% | Neutral, acidic soils | 1.2 |
| Heptahydrate Magnesium Sulfate | Fast | 80%–85% | Neutral, acidic soils | 1 |
| Magnesium Oxide | Slow | 40%–50% | Acidic soils | 0.8 |
| Magnesium Chloride | Fast | 75%–80% | Neutral soils | 1.1 |
Application Methods:
- Foliar Spray: 0.3%–0.5% solution, applied every 7–10 days for fruit trees (e.g., citrus, grapes).
- Soil Application: 5–8 kg per acre, mixed with organic fertilizer, avoiding direct seed contact to prevent seedling burn.
3.5 Emerging Applications (2% Share)
1. New Energy Materials
- Lithium Battery Electrolyte Additive: Adding 0.1%–0.5% magnesium sulfate anhydrous to electrolyte suppresses HF formation (which corrodes electrodes), extending battery cycle life (from 1,000 to 1,500 cycles).
- Sodium-Sulfur Battery Cathode: Compounded with sulfur (MgSO₄:S = 1:3) to improve sulfur conductivity, increasing battery capacity (specific capacity up to 600 mAh/g).
2. Cosmetics
- Oil Control Agent: Added to facial cleansers and toners (0.5%–1%) to shrink sebaceous gland pores, reducing oil secretion (effect lasts 4–6 hours).
- Stabilizer: Used in emulsions and creams to prevent oil-water separation, extending shelf life (from 6 to 12 months).
4. Safety and Storage: Risk Control and Standard Operations
4.1 Hazard Identification
- Classification: Not listed in the 2022 Hazardous Chemicals Catalog; considered a general chemical but poses the following risks:
- Dust Irritation: Prolonged inhalation of dust may irritate respiratory mucosa, causing coughing or chest tightness (LD₅₀ oral rat > 6450 mg/kg, low toxicity).
- Skin Irritation: Direct contact with dry skin is non-irritating, but prolonged exposure to its solution (neutral) may cause dryness or peeling.
- Ingestion Risk: Adult ingestion > 20 g may cause diarrhea or vomiting; children ingesting > 5 g require immediate medical attention.
4.2 Personal Protective Equipment (PPE) and Workplace Requirements
PPE:
- Respiratory: Wear N95 dust masks (per GB 2890-2009) during dust operations; use powered air-purifying respirators in high-dust environments.
- Hands: Wear nitrile gloves (thickness ≥ 0.1 mm) to prevent solution penetration after moisture absorption.
- Eyes: Wear impact-resistant goggles to prevent dust splash.
- Body: Wear anti-static workwear in dusty environments to avoid dust accumulation.
Workplace Requirements:
- Ventilation: Ensure workshop ventilation ≥ 3 air changes/hour; install dust collection systems (air volume ≥ 1000 m³/h) at dust-generating points (e.g., pulverizing, packaging).
- Humidity Control: Maintain RH ≤ 40% to prevent clumping.
- Fire/Explosion Prevention: Non-flammable and non-explosive, but keep away from open flames to avoid decomposition at high temperatures.
4.3 Storage Conditions and Shelf Life
Storage Environment:
- Temperature: 15–30°C (avoid high temperatures causing particle sintering).
- Humidity: RH ≤ 30% (critical, use dehumidifiers).
- Light: Store in dark conditions to avoid UV-accelerated moisture absorption.
Packaging and Containers:
- Small Packaging (500 g/1 kg): Aluminum-plastic composite bags (PE inner layer, aluminum foil outer layer) with heat-sealed edges and one-way exhaust valves.
- Medium Packaging (25 kg): PE plastic drums (thickness ≥ 1.5 mm) with screw caps and nitrile sealing rings.
- Large Packaging (1000 kg): Flexible intermediate bulk containers (PP woven outer bag, PE inner liner) with sealed inner membranes.
Isolation Requirements:
- Store separately from water, acids, and alkalis (≥ 1 m distance).
- Keep away from food and pharmaceuticals to avoid cross-contamination.
Shelf Life:
- Unopened: Industrial grade: 1 year; pharmaceutical/reagent grade: 2 years (higher purity increases moisture sensitivity).
- Opened: Seal immediately and use within 1 month (moisture absorption reduces purity, impairing desiccant or fertilizer efficacy).
4.4 Emergency and Waste Handling
Spill Handling:
- Solid Spills: Sweep with plastic brooms (avoid metal tools to prevent sparks), collect in sealed bags labeled “Magnesium Sulfate Anhydrous Waste.”
- Dust Dispersion: Activate dust collection systems, evacuate personnel to upwind areas, and clean after dust settles.
- Clumped Material: Crush clumped material and dry at 200–250°C for reuse (industrial grade); pharmaceutical/reagent grades must be discarded if moist.
Fire Handling:
- Non-combustible, but if a fire occurs nearby, extinguish with water and relocate containers to prevent decomposition into toxic SO₃ gas.
Waste Handling:
- Used Desiccant (Heptahydrate after Absorption): Can be repurposed as agricultural magnesium fertilizer (heavy metal content ≤ 0.001%).
- Production Waste (e.g., CaSO₄, Fe(OH)₃): Hand over to hazardous waste disposal agencies (per GB 18599-2020).
- Wastewater: Neutralize to pH 6–9 before discharge (per GB 8978-1996).
5. Purity and Quality Control: Standards and Identification Methods
5.1 Purity Grades and Standard Requirements
| Purity Grade | Applications | MgSO₄ Content | Impurity Limits (≤) | Standard |
|---|---|---|---|---|
| Industrial | Desiccants, flame retardants | 98%–99% | Ca²⁺: 0.3%, Fe³⁺: 0.01%, Cl⁻: 0.1% | GB/T 26568-2011 |
| Food Grade | Food moisture protection, additives | ≥ 99.0% | Pb: 0.0005%, As: 0.0002%, Hg: 0.0001% | GB 25588-2010 |
| Pharmaceutical | Oral laxatives, formulations | ≥ 99.5% | Pb: 0.0001%, As: 0.00005%, Total Heavy Metals: 0.001% | ChP 2020, USP 43 |
| Reagent AR | Laboratory analysis | ≥ 99.5% | Ca²⁺: 0.02%, Fe³⁺: 0.0005%, Cl⁻: 0.001% | GB/T 671-2014 |
| Reagent GR | Precision analysis | ≥ 99.9% | Ca²⁺: 0.005%, Fe³⁺: 0.0001%, Cl⁻: 0.0005% | GB/T 671-2014 |
| Ultra-Pure | New energy materials | ≥ 99.99% | Total Metal Impurities: 0.001% | Enterprise Standard (e.g., Q/370683BYS 001-2024) |
5.2 Purity Identification Methods
1. Physical Identification (Preliminary)
- Water Absorption Test: Place 1 g sample in a petri dish at RH 60% for 1 hour; weight gain ≥ 0.1 g and surface clumping indicate magnesium sulfate anhydrous(heptahydrate gains ≤ 0.02 g).
- Dissolution Clarity: Dissolve 5 g in 100 mL deionized water; a clear, transparent solution indicates purity ≥ 98% (impurities cause turbidity).
- Density Test: Measure density with a pycnometer; 2.66 ± 0.02 g/cm³ confirms anhydrous form (heptahydrate: 1.68 g/cm³).
2. Chemical Identification (Characteristic Reactions)
- Sulfate Test: Add BaCl₂ to a sample solution; white BaSO₄ precipitate (insoluble in dilute HCl) confirms SO₄²⁻.
- Magnesium Test: Add NaOH to a sample solution; white Mg(OH)₂ precipitate (insoluble in excess NaOH, unlike Al³⁺) confirms Mg²⁺.
- Impurity Test: Add AgNO₃; no white AgCl precipitate indicates Cl⁻ ≤ 0.001%. Add KSCN; no red color indicates Fe³⁺ ≤ 0.0005%.
3. Professional Testing (Precise Analysis)
- Purity Determination: Use EDTA complexometric titration (per GB/T 671-2014) to titrate Mg²⁺ and calculate MgSO₄ content.
- Impurity Analysis: Use ICP-MS (inductively coupled plasma mass spectrometry) to measure heavy metal content (detection limit 0.00001%).
- Crystal Structure Confirmation: Use XRD (X-ray diffraction) to match characteristic peaks with magnesium sulfate anhydrous standard (JCPDS 01-071-2396), confirming no crystal water.
5.3 Procurement Priority Indicators
- Pharmaceutical/Food Grade: Heavy metal content > MgSO₄ purity > moisture > particle size (heavy metals are critical for safety).
- Reagent Grade: MgSO₄ purity > impurity content (Ca²⁺, Fe³⁺) > clarity > moisture (sensitive to purity and impurities for analysis).
- Industrial Grade: MgSO₄ purity > moisture > particle size > impurity content (desiccants and flame retardants prioritize purity and hygroscopicity).
- New Energy Grade: Total metal impurities > MgSO₄ purity > moisture > particle size distribution (batteries are highly sensitive to metal impurities causing short circuits).
6. Market and Development: Global Landscape and Trends
6.1 Market Size and Regional Distribution (2024 Data)
Global Market:
- Annual Production: ~300,000 tons; consumption: ~280,000 tons; growth rate: 5%–7%.
- Main Regions: China (60%, ~180,000 tons), North America (15%, ~45,000 tons), Europe (12%, ~36,000 tons), others (13%).
- Application Structure: Desiccants (40%), industrial (35%), pharmaceuticals (15%), agriculture (8%), emerging fields (2%).
China Market:
- Capacity: ~350,000 tons; production: 180,000 tons; consumption: 160,000 tons (exports: 20,000 tons, mainly to Southeast Asia and Europe).
- Main Production Areas: Shandong (Laizhou, Weifang, 40%), Qinghai (salt lake region, 30%), Jiangsu (fine chemicals, 20%), others (10%).
Crystal Form Market Share:
- Heptahydrate (70%) > Anhydrous (15%) = Monohydrate (15%). Magnesium sulfate anhydrous has a lower share due to higher costs (30%–50% more than heptahydrate) but grows faster (8%–10% annually).
6.2 Major Companies and Competitiveness
| Company | Region | Capacity (Tons/Year) | Core Product Purity | Core Competitiveness | 2024 Avg. Price (CNY/Ton) |
|---|---|---|---|---|---|
| Shandong Laizhou Baiye Chemical | China | 100,000 | Industrial: 98%–99%, Food: 99.5% | High-quality raw materials, low cost, customized services | 1,800–2,500 |
| PQ Corporation | USA | 40,000 | Reagent: 99.9%, Pharmaceutical: 99.5% | Advanced technology, robust global supply chain | 6,000–8,000 |
| BASF SE | Germany | 30,000 | Industrial: 99%, Food: 99.5% | Eco-friendly processes, high-end market advantage | 5,500–7,000 |
| K+S Group | Germany | 100,000 | Industrial: 98%–99%, Pharmaceutical: 99.5% | Strong global supply chain, industrial focus | 5,500–7,000 |
| Laizhou Laiyu | China | 60,000 | Industrial: 98% | Resource advantages, high purity | 2,200–2,800 |
| Kanto Chemical | Japan | 10,000 | Reagent: 99.99% | High-purity products, suited for electronics | 15,000–20,000 |
Price Influencing Factors:
- Raw Material Costs: Sulfuric acid (20% of cost), magnesium oxide (30% for pharmaceutical grade) price fluctuations.
- Supply and Demand: Prices rise 5%–10% in peak desiccant demand seasons (summer, high humidity).
- Purity and Form: Each 0.5% purity increase raises prices by 10%–15%; granules are 15%–20% more expensive than powders.
6.3 Technology Trends and Industry Opportunities/Challenges
Technology Trends:
- Low-Energy Dehydration: Solar-assisted calcination reduces energy use by 30% (from 800 kW·h/t to 560 kW·h/t).
- High-Purity Production: Membrane separation + ion exchange achieves 99.99% ultra-pure products for new energy applications.
- Functional Modification: Magnesium sulfate anhydrous-molecular sieve composites increase water absorption by 50% and lower regeneration temperature to 150°C.
- Eco-Friendly Processes: Zero wastewater discharge (via evaporative crystallization) and waste slag repurposing (e.g., for gypsum boards) promote resource recycling.
Opportunities:
- Emerging Fields: Lithium battery electrolyte additive demand grows >30% annually, projected to exceed 10,000 tons by 2025.
- Environmental Policies: EU REACH regulations restrict chloride-based desiccants (e.g., calcium chloride), boosting magnesium sulfate anhydrous substitution.
- Agricultural Upgrades: Expanded cultivation of high-value crops (e.g., organic grapes, citrus) increases demand for efficient magnesium fertilizers.
Challenges:
- Cost Pressure: Sulfuric acid price fluctuations (20%–30% annually, e.g., from 500 to 700 CNY/ton in 2024).
- Substitution Competition: Molecular sieves replace magnesium sulfate anhydrous in electronics (despite higher costs, better reusability).
- Environmental Requirements: China’s “dual carbon” goals require calcination process emission reductions (e.g., carbon capture systems), increasing costs.
7. Packaging Specifications
| Packaging Type | Specification | Material | Applications | Moisture-Proof Features |
|---|---|---|---|---|
| Small Packaging | 500 g/1 kg | Aluminum-plastic composite bag (PE inner, aluminum foil outer) | Lab reagents, small-scale use | Heat-sealed edges, one-way exhaust valve |
| Medium Packaging | 25 kg | PE plastic drum (1.5 mm thick) | Industrial, food/pharmaceutical raw materials | Screw cap with nitrile sealing ring |
| Large Packaging | 1000 kg | Flexible intermediate bulk container (PP woven outer, PE inner liner) | Bulk industrial procurement | Sealed inner membrane, waterproof cover |
| Custom Packaging | 10 g/20 g | Non-woven breathable bag (PE inner film) | Pharmaceutical moisture-proof sachets | Breathable, dust-proof, controlled absorption rate |
8. Frequently Asked Questions (FAQ)
1. Are magnesium sulfate anhydrous and heptahydrate magnesium sulfate the same substance? Can they be interconverted? They are not the same due to differing crystal water content but can be interconverted:
- Anhydrous to Heptahydrate: Anhydrous form absorbs moisture at RH > 60% or dissolves in water and crystallizes upon cooling.
- Heptahydrate to Anhydrous: Dehydrate heptahydrate at 200–250°C.
2. What happens to magnesium sulfate anhydrous after absorbing water? Does it retain value? It first forms monohydrate (powder), then heptahydrate (clumps). Industrial-grade products can be regenerated by drying at 200–250°C; pharmaceutical/reagent grades lose purity and must be discarded.
3. Is the solution composition identical after dissolving anhydrous and heptahydrate magnesium sulfate in water? Yes, both dissociate into Mg²⁺ and SO₄²⁻. However, due to crystal water, 10 g magnesium sulfate anhydrous in 100 mL water yields ~0.83 mol/L Mg²⁺, while 10 g heptahydrate yields ~0.4 mol/L.
4. Is magnesium sulfate anhydrous safe for pregnant women and children?
- Pregnant Women: Oral use requires medical supervision (used intravenously for pre-eclampsia; oral use may cause dehydration, affecting the fetus). Topical use (in ointments) is safe.
- Children: Oral dose is halved (e.g., 1–2 g vs. 2–5 g for adults) to avoid electrolyte imbalances. Desiccants should not be handled by children to prevent ingestion.
5. Can magnesium sulfate anhydrous desiccant be reused? What are the regeneration conditions? Yes (industrial grade only). Regenerate by drying moist desiccant (monohydrate/heptahydrate) at 200–250°C for 2–3 hours until initial weight is restored, then seal for storage.
6. How to distinguish anhydrous from heptahydrate magnesium sulfate?
- Weight Method: Anhydrous is 1.6 times heavier than heptahydrate for the same volume (density difference).
- Heating Method: Heat 1 g to 200°C; anhydrous weight remains stable, heptahydrate loses ~51% (crystal water).
- Solubility: 10 g anhydrous fully dissolves in 100 mL water; heptahydrate may leave crystals due to supersaturation.
7. Why does magnesium sulfate anhydrous clump during storage, and how to address it?
- Cause: Storage humidity > 30% or poor sealing causes moisture absorption.
- Solutions:
- Prevention: Maintain RH ≤ 30%, use sealed packaging.
- Treatment: Crush light clumps for industrial use; dry severe clumps at 200–250°C for regeneration.
8. Is magnesium sulfate anhydrous polar or nonpolar? It is polar. As an ionic crystal, it dissolves in polar solvents like water (forming coordination bonds with Mg²⁺ and SO₄²⁻) but is insoluble in nonpolar solvents (e.g., benzene, ether).
9. What is the molar mass of magnesium sulfate anhydrous ? How to calculate its mass?
- Molar Mass: 120.37 g/mol (Mg: 24.31 + S: 32.07 + 4×O: 16.00 = 120.37).
- Mass Calculation: Mass (g) = moles × 120.37 g/mol. E.g., 0.5 mol = 0.5 × 120.37 = 60.185 g.
10. Why is magnesium sulfate anhydrous used to dry organic layers?
- Reasons:
- Insoluble in organic layers, avoiding contamination.
- Strong water absorption, quickly removes trace moisture.
- Neutral, does not react with organic compounds (e.g., esters, ketones, hydrocarbons).
- Easily filtered, unlike molecular sieves, which may cause dust pollution.
Magnesium Sulfate Anhydrous -BAIYEXIN

Baiyexin is the largest Magnesium Sulfate Anhydrous - manufacturer in China, providing Magnesium Sulfate Anhydrous - in various specifications.
Product SKU: BYX6012
Product Brand: BAIYEXIN
Product Currency: USD
Product Price: 260-320
Price Valid Until: 2025-12-31
Product In-Stock: InStock
5






BaiYeXin –
What we value most is that it is genuinely supplied directly by the manufacturer. Magnesium Sulfate Anhydrous has a stable pricing system that avoids cutthroat competition, making it suitable for long-term distribution.